How to make an electronic formula for chemistry. Chemical formulas of substances. Examples of compiling electronic formulas
Cheat sheet with formulas in physics for the Unified State Exam
and more (may be needed for grades 7, 8, 9, 10 and 11).
First, a picture that can be printed in a compact form.
Mechanics
- Pressure P=F/S
- Density ρ=m/V
- Pressure at liquid depth P=ρ∙g∙h
- Gravity Ft=mg
- 5. Archimedean force Fa=ρ f ∙g∙Vt
- Equation of motion for uniformly accelerated motion
X=X 0 + υ 0 ∙t+(a∙t 2)/2 S=( υ 2 -υ 0 2) /2a S=( υ +υ 0) ∙t /2
- Velocity equation for uniformly accelerated motion υ =υ 0 +a∙t
- Acceleration a=( υ -υ 0)/t
- Circular speed υ =2πR/T
- Centripetal acceleration a= υ 2/R
- Relationship between period and frequency ν=1/T=ω/2π
- Newton's II law F=ma
- Hooke's law Fy=-kx
- Law of Gravity F=G∙M∙m/R 2
- Weight of a body moving with acceleration a P=m(g+a)
- Weight of a body moving with acceleration а↓ Р=m(g-a)
- Friction force Ftr=µN
- Body momentum p=m υ
- Force impulse Ft=∆p
- Moment of force M=F∙ℓ
- Potential energy of a body raised above the ground Ep=mgh
- Potential energy of an elastically deformed body Ep=kx 2 /2
- Kinetic energy of the body Ek=m υ 2 /2
- Work A=F∙S∙cosα
- Power N=A/t=F∙ υ
- Efficiency η=Ap/Az
- Oscillation period of a mathematical pendulum T=2π√ℓ/g
- Oscillation period of a spring pendulum T=2 π √m/k
- Equation of harmonic vibrations Х=Хmax∙cos ωt
- Relationship between wavelength, its speed and period λ= υ T
Molecular physics and thermodynamics
- Amount of substance ν=N/Na
- Molar mass M=m/ν
- Wed. kin. energy of monatomic gas molecules Ek=3/2∙kT
- Basic MKT equation P=nkT=1/3nm 0 υ 2
- Gay-Lussac's law (isobaric process) V/T =const
- Charles's law (isochoric process) P/T =const
- Relative humidity φ=P/P 0 ∙100%
- Int. energy ideal. monatomic gas U=3/2∙M/µ∙RT
- Gas work A=P∙ΔV
- Boyle–Mariotte law (isothermal process) PV=const
- Amount of heat during heating Q=Cm(T 2 -T 1)
- Amount of heat during melting Q=λm
- Amount of heat during vaporization Q=Lm
- Amount of heat during fuel combustion Q=qm
- Equation of state of an ideal gas PV=m/M∙RT
- First law of thermodynamics ΔU=A+Q
- Efficiency of heat engines η= (Q 1 - Q 2)/ Q 1
- Efficiency is ideal. engines (Carnot cycle) η= (T 1 - T 2)/ T 1
Electrostatics and electrodynamics - formulas in physics
- Coulomb's law F=k∙q 1 ∙q 2 /R 2
- Electric field strength E=F/q
- Electrical tension point charge field E=k∙q/R 2
- Surface charge density σ = q/S
- Electrical tension fields of an infinite plane E=2πkσ
- Dielectric constant ε=E 0 /E
- Potential energy interaction. charges W= k∙q 1 q 2 /R
- Potential φ=W/q
- Point charge potential φ=k∙q/R
- Voltage U=A/q
- For a uniform electric field U=E∙d
- Electric capacity C=q/U
- Electric capacity of a flat capacitor C=S∙ ε ∙ε 0 /d
- Energy of a charged capacitor W=qU/2=q²/2С=CU²/2
- Current strength I=q/t
- Conductor resistance R=ρ∙ℓ/S
- Ohm's law for the circuit section I=U/R
- Laws of the last. connections I 1 =I 2 =I, U 1 +U 2 =U, R 1 +R 2 =R
- Laws parallel. conn. U 1 =U 2 =U, I 1 +I 2 =I, 1/R 1 +1/R 2 =1/R
- Electric current power P=I∙U
- Joule-Lenz law Q=I 2 Rt
- Ohm's law for a complete circuit I=ε/(R+r)
- Short circuit current (R=0) I=ε/r
- Magnetic induction vector B=Fmax/ℓ∙I
- Ampere power Fa=IBℓsin α
- Lorentz force Fl=Bqυsin α
- Magnetic flux Ф=BSсos α Ф=LI
- Law of electromagnetic induction Ei=ΔФ/Δt
- Induction emf in a moving conductor Ei=Вℓ υ sinα
- Self-induction EMF Esi=-L∙ΔI/Δt
- Coil magnetic field energy Wm=LI 2 /2
- Oscillation period no. circuit T=2π ∙√LC
- Inductive reactance X L =ωL=2πLν
- Capacitance Xc=1/ωC
- Effective current value Id=Imax/√2,
- Effective voltage value Uд=Umax/√2
- Impedance Z=√(Xc-X L) 2 +R 2
Optics
- Law of light refraction n 21 =n 2 /n 1 = υ 1 / υ 2
- Refractive index n 21 =sin α/sin γ
- Thin lens formula 1/F=1/d + 1/f
- Lens optical power D=1/F
- max interference: Δd=kλ,
- min interference: Δd=(2k+1)λ/2
- Differential grid d∙sin φ=k λ
Quantum physics
- Einstein's formula for the photoelectric effect hν=Aout+Ek, Ek=U z e
- Red border of the photoelectric effect ν k = Aout/h
- Photon momentum P=mc=h/ λ=E/s
Physics of the atomic nucleus
- Law of radioactive decay N=N 0 ∙2 - t / T
- Binding energy of atomic nuclei
Chemical formula is an image using symbols.
Chemical element signs
Chemical sign or chemical element symbol– this is the first or two first letters of the Latin name of this element.
For example: FerrumFe , Cuprum –Cu , OxygeniumO etc.
Table 1: Information provided by a chemical symbol
| Intelligence | Using the example of Cl |
| Item name | Chlorine |
| Non-metal, halogen | |
| One element | 1 chlorine atom |
| (Ar) of this element | Ar(Cl) = 35.5 |
| Absolute atomic mass of a chemical element
m = Ar 1.66 10 -24 g = Ar 1.66 10 -27 kg |
M (Cl) = 35.5 1.66 10 -24 = 58.9 10 -24 g |
The name of a chemical symbol in most cases is read as the name of a chemical element. For example, K – potassium, Ca – calcium, Mg – magnesium, Mn – manganese.
Cases when the name of a chemical symbol is read differently are given in Table 2:
| Chemical element name | Chemical sign | Chemical symbol name
(pronunciation) |
| Nitrogen | N | En |
| Hydrogen | H | Ash |
| Iron | Fe | Ferrum |
| Gold | Au | Aurum |
| Oxygen | O | ABOUT |
| Silicon | Si | Silicium |
| Copper | Cu | Cuprum |
| Tin | Sn | Stanum |
| Mercury | Hg | Hydrargium |
| Lead | Pb | Plumbum |
| Sulfur | S | Es |
| Silver | Ag | Argentum |
| Carbon | C | Tse |
| Phosphorus | P | Pe |
Chemical formulas of simple substances
The chemical formulas of most simple substances (all metals and many non-metals) are the signs of the corresponding chemical elements.
So iron substance And chemical element iron are designated the same - Fe .
If it has a molecular structure (exists in the form , then its formula is the chemical sign of the element with index bottom right indicating number of atoms in a molecule: H 2, O2, O 3, N 2, F 2, Cl2, BR 2, P 4, S 8.
Table 3: Information provided by a chemical sign
| Intelligence | Using C as an example |
| Substance name | Carbon (diamond, graphite, graphene, carbyne) |
| Belonging of an element to a given class of chemical elements | Non-metal |
| One atom of an element | 1 carbon atom |
| Relative atomic mass (Ar) element that forms a substance | Ar(C) = 12 |
| Absolute atomic mass | M(C) = 12 1.66 10-24 = 19.93 10 -24 g |
| One substance | 1 mole of carbon, i.e. 6.02 10 23 carbon atoms |
| M (C) = Ar (C) = 12 g/mol |
Chemical formulas of complex substances
The formula of a complex substance is prepared by writing down the signs of the chemical elements of which the substance is composed, indicating the number of atoms of each element in the molecule. In this case, as a rule, chemical elements are written in order of increasing electronegativity in accordance with the following practical series:
Me, Si, B, Te, H, P, As, I, Se, C, S, Br, Cl, N, O, F
For example, H2O , CaSO4 , Al2O3 , CS 2 , OF 2 , NaH.
The exceptions are:
- some compounds of nitrogen with hydrogen (for example, ammonia NH 3 , hydrazine N 2H 4 );
- salts of organic acids (for example, sodium formate HCOONa , calcium acetate (CH 3COO) 2Ca) ;
- hydrocarbons ( CH 4 , C2H4 , C2H2 ).
Chemical formulas of substances existing in the form dimers (NO 2 , P2O 3 , P2O5, salts of monovalent mercury, for example: HgCl , HgNO3 etc.), written in the form N 2 O4,P 4 O6,P 4 O 10Hg 2 Cl2,Hg 2 ( NO 3) 2 .
The number of atoms of a chemical element in a molecule and a complex ion is determined based on the concept valency or oxidation states and is recorded index lower right from the sign of each element (index 1 is omitted). In this case, they proceed from the rule:
the algebraic sum of the oxidation states of all atoms in a molecule must be equal to zero (the molecules are electrically neutral), and in a complex ion - the charge of the ion.
For example:
2Al 3 + +3SO 4 2- =Al 2 (SO 4) 3
The same rule is used when determining the oxidation state of a chemical element using the formula of a substance or complex. It is usually an element that has several oxidation states. The oxidation states of the remaining elements forming the molecule or ion must be known.
The charge of a complex ion is the algebraic sum of the oxidation states of all the atoms that form the ion. Therefore, when determining the oxidation state of a chemical element in a complex ion, the ion itself is placed in brackets, and its charge is taken out of brackets.
When compiling formulas for valency a substance is represented as a compound consisting of two particles of different types, the valencies of which are known. Next they use rule:
in a molecule, the product of valence by the number of particles of one type must be equal to the product of valence by the number of particles of another type.
For example:
The number before the formula in a reaction equation is called coefficient. She indicates either number of molecules, or number of moles of substance.
The coefficient before the chemical symbol, indicates number of atoms of a given chemical element, and in the case when the sign is the formula of a simple substance, the coefficient indicates either number of atoms, or the number of moles of this substance.
For example:
- 3 Fe– three iron atoms, 3 moles of iron atoms,
- 2 H– two hydrogen atoms, 2 moles of hydrogen atoms,
- H 2– one molecule of hydrogen, 1 mole of hydrogen.
The chemical formulas of many substances have been determined experimentally, which is why they are called "empirical".
Table 4: Information provided by the chemical formula of a complex substance
| Intelligence | Using the example C aCO3 |
| Substance name | Calcium carbonate |
| Belonging of an element to a certain class of substances | Medium (normal) salt |
| One molecule of substance | 1 molecule calcium carbonate |
| One mole of substance | 6.02 10 23 molecules CaCO3 |
| Relative molecular mass of the substance (Mr) | Мr (CaCO3) = Ar (Ca) +Ar (C) +3Ar (O) =100 |
| Molar mass of the substance (M) | M (CaCO3) = 100 g/mol |
| Absolute molecular mass of the substance (m) | M (CaCO3) = Mr (CaCO3) 1.66 10 -24 g = 1.66 10 -22 g |
| Qualitative composition (what chemical elements form the substance) | calcium, carbon, oxygen |
| Quantitative composition of the substance: | |
| The number of atoms of each element in one molecule of a substance: | a calcium carbonate molecule is made up of 1 atom calcium, 1 atom carbon and 3 atoms oxygen. |
| The number of moles of each element in 1 mole of the substance: | In 1 mole CaCO 3(6.02 · 10 23 molecules) contained 1 mole(6.02 · 10 23 atoms) calcium, 1 mole(6.02 10 23 atoms) carbon and 3 mol(3 6.02 10 23 atoms) of the chemical element oxygen) |
| Mass composition of the substance: | |
| Mass of each element in 1 mole of substance: | 1 mole of calcium carbonate (100g) contains the following chemical elements: 40g calcium, 12g carbon, 48g oxygen. |
| Mass fractions of chemical elements in the substance (composition of the substance as a percentage by weight):
|
Composition of calcium carbonate by weight:
W (Ca) = (n (Ca) Ar (Ca))/Mr (CaCO3) = (1·40)/100= 0.4 (40%) W (C) = (n (Ca) Ar (Ca))/Mr (CaCO3) = (1 12)/100 = 0.12 (12%) W (O) = (n (Ca) Ar (Ca))/Mr (CaCO3) = (3 16)/100 = 0.48 (48%) |
| For a substance with an ionic structure (salt, acid, base), the formula of the substance provides information about the number of ions of each type in the molecule, their quantity and the mass of ions per 1 mole of the substance:
|
Molecule CaCO 3 consists of an ion Ca 2+ and ion CO 3 2-
1 mol ( 6.02 10 23 molecules) CaCO 3 contains 1 mol Ca 2+ ions And 1 mole ions CO 3 2-; 1 mole (100g) of calcium carbonate contains 40g ions Ca 2+ And 60g ions CO 3 2- |
| Molar volume of a substance at standard conditions (for gases only) | |
Graphic formulas
To obtain more complete information about a substance, use graphic formulas , which indicate order of connection of atoms in a molecule And valence of each element.
Graphic formulas of substances consisting of molecules sometimes, to one degree or another, reflect the structure (structure) of these molecules; in these cases they can be called structural .
To compile a graphical (structural) formula of a substance, you must:
- Determine the valence of all chemical elements that form the substance.
- Write down the signs of all chemical elements that form the substance, each in an amount equal to the number of atoms of a given element in the molecule.
- Connect the signs of chemical elements with dashes. Each dash denotes a pair that communicates between chemical elements and therefore belongs equally to both elements.
- The number of lines surrounding the sign of a chemical element must correspond to the valency of this chemical element.
- When formulating oxygen-containing acids and their salts, hydrogen atoms and metal atoms are bonded to the acid-forming element through an oxygen atom.
- Oxygen atoms are combined with each other only when formulating peroxides.
Examples of graphic formulas:
Let's find out how to create the electronic formula of a chemical element. This question is important and relevant, as it gives an idea not only of the structure, but also of the expected physical and chemical properties of the atom in question.
Compilation rules
In order to compose a graphical and electronic formula of a chemical element, it is necessary to have an understanding of the theory of atomic structure. To begin with, there are two main components of an atom: the nucleus and the negative electrons. The nucleus includes neutrons, which have no charge, as well as protons, which have a positive charge.
Discussing how to compose and determine the electronic formula of a chemical element, we note that to find the number of protons in the nucleus, the Mendeleev periodic system will be required.
The number of an element corresponds in order to the number of protons found in its nucleus. The number of the period in which the atom is located characterizes the number of energy layers on which electrons are located.
To determine the number of neutrons devoid of electrical charge, it is necessary to subtract its serial number (number of protons) from the relative mass of an element’s atom.
Instructions
In order to understand how to compose the electronic formula of a chemical element, consider the rule for filling sublevels with negative particles, formulated by Klechkovsky.
Depending on how much free energy the free orbitals have, a series is compiled that characterizes the sequence of filling the levels with electrons.
Each orbital contains only two electrons, which are arranged in antiparallel spins.
In order to express the structure of electronic shells, graphic formulas are used. What do the electronic formulas of atoms of chemical elements look like? How to create graphic options? These questions are included in the school chemistry course, so we will dwell on them in more detail.
There is a certain matrix (basis) that is used when drawing up graphic formulas. The s-orbital is characterized by only one quantum cell, in which two electrons are located opposite each other. They are indicated graphically by arrows. For the p-orbital, three cells are depicted, each also containing two electrons, the d orbital contains ten electrons, and the f orbital is filled with fourteen electrons.

Examples of compiling electronic formulas
Let's continue the conversation about how to compose the electronic formula of a chemical element. For example, you need to create a graphical and electronic formula for the element manganese. First, let's determine the position of this element in the periodic table. It has atomic number 25, therefore, there are 25 electrons in the atom. Manganese is a fourth period element and therefore has four energy levels.
How to write the electronic formula of a chemical element? We write down the sign of the element, as well as its serial number. Using Klechkovsky’s rule, we distribute electrons among energy levels and sublevels. We place them sequentially on the first, second, and third levels, placing two electrons in each cell.
Next, we sum them up, getting 20 pieces. Three levels are completely filled with electrons, and only five electrons remain on the fourth. Considering that each type of orbital has its own energy reserve, we distribute the remaining electrons into the 4s and 3d sublevels. As a result, the finished electronic graphic formula for the manganese atom has the following form:
1s2 / 2s2, 2p6 / 3s2, 3p6 / 4s2, 3d3

Practical significance
Using electron graphic formulas, you can clearly see the number of free (unpaired) electrons that determine the valence of a given chemical element.
We offer a generalized algorithm of actions with which you can create electron graphic formulas for any atoms located in the periodic table.
First of all, it is necessary to determine the number of electrons using the periodic table. The period number indicates the number of energy levels.
Belonging to a certain group is associated with the number of electrons located in the outer energy level. The levels are divided into sublevels and filled in taking into account the Klechkovsky rule.

Conclusion
In order to determine the valence possibilities of any chemical element located in the periodic table, it is necessary to compile an electronic graphic formula of its atom. The algorithm given above will allow us to cope with the task and determine the possible chemical and physical properties of the atom.
Algorithm for composing the electronic formula of an element:
1. Determine the number of electrons in an atom using the Periodic Table of Chemical Elements D.I. Mendeleev.
2. Using the number of the period in which the element is located, determine the number of energy levels; the number of electrons in the last electronic level corresponds to the group number.
3. Divide the levels into sublevels and orbitals and fill them with electrons in accordance with the rules for filling orbitals:
It must be remembered that the first level contains a maximum of 2 electrons 1s 2, on the second - a maximum of 8 (two s and six r: 2s 2 2p 6), on the third - a maximum of 18 (two s, six p, and ten d: 3s 2 3p 6 3d 10).
- Principal quantum number n should be minimal.
- First to fill s- sublevel, then р-, d- b f- sublevels.
- Electrons fill the orbitals in order of increasing energy of the orbitals (Klechkovsky's rule).
- Within a sublevel, electrons first occupy free orbitals one by one, and only after that they form pairs (Hund’s rule).
- There cannot be more than two electrons in one orbital (Pauli principle).
Examples.
1. Let's create an electronic formula for nitrogen. Nitrogen is number 7 on the periodic table.

2. Let's create the electronic formula for argon. Argon is number 18 on the periodic table.
1s 2 2s 2 2p 6 3s 2 3p 6.

3. Let's create the electronic formula of chromium. Chromium is number 24 on the periodic table.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 5

Energy diagram of zinc.
4. Let's create the electronic formula of zinc. Zinc is number 30 on the periodic table.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10
Please note that part of the electronic formula, namely 1s 2 2s 2 2p 6 3s 2 3p 6, is the electronic formula of argon.
The electronic formula of zinc can be represented as:
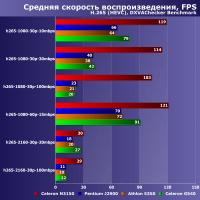 Key features of the new codec
Key features of the new codec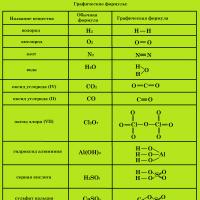 Chemical formulas of substances
Chemical formulas of substances Punctuation marks for direct speech
Punctuation marks for direct speech Step-by-step recipe for making pork liver cutlets
Step-by-step recipe for making pork liver cutlets Light fitness salad with Chinese cabbage and bell pepper Chinese cabbage salad with red pepper
Light fitness salad with Chinese cabbage and bell pepper Chinese cabbage salad with red pepper Soft chocolate processed cheese
Soft chocolate processed cheese Nekrasov's poem "Grandfather": analysis and characteristics of the work
Nekrasov's poem "Grandfather": analysis and characteristics of the work