The main methods for obtaining nanomaterials briefly. Basic technologies for obtaining nanomaterials. Consolidated Materials Technology
The structure and, accordingly, the properties of nanomaterials are formed at the stage of their manufacture. The importance of technology as a basis for ensuring stable and optimal performance of nanomaterials is quite obvious; this is also important from the point of view of their economy.
The technology of nanomaterials, in accordance with the diversity of the latter, is characterized by a combination, on the one hand, of metallurgical, physical, chemical, and biological methods, and, on the other hand, of traditional and fundamentally new methods. So, if the vast majority of methods for obtaining consolidated nanomaterials are quite traditional, then such operations as manufacturing, for example, "quantum pens" using a scanning tunneling microscope, the formation of quantum dots by self-assembly of atoms, or the use of ion-track technology to create porous structures in polymer materials are based on fundamentally different technological methods.
The methods of molecular biotechnology are also very diverse. All this complicates the presentation of the fundamentals of nanomaterial technology, taking into account the fact that many technological details (“know-how”) are described by the authors only in general terms, and often the message is of an advertising nature. Further, only the main and most characteristic technological methods are analyzed.
Consolidated Materials Technology
Powder technology
A powder is understood as a set of individual solid bodies (or their aggregates) in contact with small sizes - from a few nanometers to a thousand microns [Powder materials science / Andrievsky R.A. - M.: Metallurgy, 1991. - 205 p.]. With regard to the manufacture of nanomaterials, ultrafine powders are used as raw materials; particles with a size of not more than 100 nm, as well as larger powders obtained under conditions of intensive grinding and consisting of small crystallites with a size similar to those indicated above.
The subsequent operations of powder technology - pressing, sintering, hot pressing, etc. - are designed to provide a sample (product) of given shapes and sizes with the appropriate structure and properties. The totality of these operations is often called, at the suggestion of M.Yu. Balshina, consolidation. With regard to nanomaterials, consolidation should provide, on the one hand, almost complete compaction (i.e., the absence of macro- and micropores in the structure), and, on the other hand, preserve the nanostructure associated with the initial dimensions of the ultrafine powder (i.e., the grain size in sintered materials should be as small as possible and in any case less than 100 nm).
Methods for obtaining powders for the manufacture of nanomaterials are very diverse; they can be conditionally divided into chemical and physical, the main ones, of which, with an indication of the most characteristic ultrafine powders, are given in Table 1.
Table 1. Main methods for obtaining powders for the manufacture of nanomaterials
|
method variant |
materials |
|
|
Physical Methods |
||
|
Evaporation and condensation |
Vacuum or inert gas |
Zn, Cu, Ni, Al, Be, Sn, Pb, Mg, Ag, Cr, MgO, Al 2 O 3 , Y 2 O 3 , ZrO 2 , SiC |
|
in the reaction gas |
TiN, AlN, ZrN, NbN, ZrO 3 , Al 2 O 3 , TiO 2 . |
|
|
High Energy Destruction |
Grinding |
Fe-Cr, Be, Al 2 O 3 , TiC, Si 3 N 4 , NiAl, TiAl, AlN |
|
Detonation treatment |
BN, SiN, TiC, Fe, diamond |
|
|
electric explosion |
Al, Cd, Al 2 O 3, TiO 2. |
|
|
Chemical Methods |
||
|
Plasma chemical |
TiC, TiN, Ti(C,N), VN, AlN, SiC, Si 3 N 4 , BN, W |
|
|
laser |
Si 3 N 4 , SiC, Si 3 N 4 -SiC |
|
|
Thermal |
Fe, Cu, Ni, Mo, W, BN, TiC, WC-Co |
|
|
Self-propagating high temperature |
SiC, MoSi2, Aln, TaC |
|
|
mechanochemical |
TiC, TiN, NiAl, TiB 2 , Fe-Cu, W-Cu |
|
|
Electrochemical |
WC, CeO 2 , ZrO 2 , WB 4 |
|
|
mortar |
Mo 2 C, BN, TiB 2 , SiC |
|
|
cryochemical |
||
|
Thermal decomposition |
Condensed Precursors |
Fe, Ni, Co, SiC, Si 3 N 4 , BN, AlN, ZrO 2 , NbN |
|
Gaseous precursors |
ZrB 2 , TiB 2 , BN |
Let us consider some of the methods for obtaining ultrafine powders.
condensation method . This method has been known for a long time and theoretically studied to the greatest extent. There are homogeneous and heterogeneous nucleation of embryos (clusters).
In the first case, the nucleus appears fluctuationally, and by changing the supersaturation of the system (increasing or decreasing the vapor pressure, varying the process temperature), one can control the value of the critical nucleus radius and achieve the required particle size of the obtained powders. By conducting evaporation in neutral media and introducing foreign surfaces into the evaporation space, it is possible to provoke heterogeneous nucleation for which the height of the potential barrier for the formation of a critical nucleus is much lower compared to bulk homogeneous condensation. Thus, there are at least two necessary and sufficient conditions for obtaining ultrafine powders by condensation methods - a large supersaturation and the presence of neutral gas molecules in the condensed vapor.
A laboratory setup for obtaining metallic ultrafine powders was developed at the Institute of Chemical Physics of the USSR Academy of Sciences in the 1960s. [Levitation method for obtaining ultradispersed metal powders /Gen M.Ya., Miller A.V. Surface. Physics, chemistry, mechanics. - 1983. No. 2., S. 150-154.]. A drop of molten metal hanging in an induction field is blown by a stream of high-purity argon, which carries the condensed nanoparticles into a special powder collector, which is unloaded in a controlled non-oxidizing atmosphere. The subsequent storage of powders and the corresponding technological operations are also carried out in argon.
The condensation method was used in the Glater installation (Figure 1), in which the production of ultrafine powder in an atmosphere of rarefied inert gas is combined with vacuum pressing. Nanoparticles condensed on the surface of a cooled rotating cylinder are removed with a special scraper and collected in a mold 2 pre-pressing (pressure up to 1 GPa), and then in a special mold 1 compaction is carried out at higher (up to 3–5 GPa) pressures. The productivity of the Glaiter plant is low, it is limited mainly by low evaporation rates.
Figure 1. Scheme of the Glaiter installation: 1 - compaction unit at high pressure; 2 - pre-compression unit; 3 - evaporator; 4 - rotating collector cooled with liquid nitrogen; 5 - scraper
Condensation methods, in principle, ensure the production of ultrafine powders with a particle size of up to several nanometers, but the duration of the process of obtaining such objects (and, accordingly, the cost) is quite large. At the request of consumers, thin polymer films can be applied to the powder surface to prevent agglomeration and corrosion.
High energy grinding . Mechanochemical synthesis . Shredding is a typical example of top-down technology. Grinding in mills, disintegrators, attritors and other dispersing devices occurs due to crushing, splitting, cutting, abrasion, sawing, impact, or a combination of these actions. Figure 2 shows the scheme of the attritor, in which, due to the rotation of the crushed charge and balls, the impact and abrasive effects are combined, and the scheme of the vibrating mill, the design of which provides a high speed of the balls and the frequency of impacts. To provoke destruction, grinding is often carried out at low temperatures. Grinding efficiency is influenced by the ratio of mass of balls and ground mixture, which is usually maintained in the range from 5:1 to 40:1.

Figure 2 Scheme of grinding plants:
a - attritor (1 - body, 2 - balls, 3 - rotating impeller); b - vibrating mill (1 - engine, 2 - vibrator, 3 - springs, 4 - drums with balls and crushed charge)
Providing, in principle, an acceptable performance, grinding, however, does not lead to the production of very fine powders, since there is a certain grinding limit corresponding to the achievement of a kind of balance between the process of particle destruction and their agglomeration. Even when grinding brittle materials, the resulting particle size is usually not less than about 100 nm; particles consist of crystallites with a size of at least 10–20 nm. It should also be taken into account that during the grinding process, the product is almost always contaminated with the material of the balls and lining, as well as with oxygen.
Plasma chemical synthesis [Troitsky V.N. Obtaining ultrafine powders in plasma microwave discharge// Microwave plasma generators: physics, technology, application/ Batenin V.M. and others - M.: Energoatomizdat, 1988. - S. 175-221.]. Synthesis in low-temperature plasma is carried out at high temperatures (up to 6000-8000 K), which ensures a high level of supersaturation, high rates of reactions and condensation processes. Both arc plasma torches and high- and microwave-frequency (SHF) plasma generators are used. Arc machines are more productive and affordable, but microwave units produce finer and purer powders. A diagram of such a setup is shown in Figure 3. Metal chlorides, metal powders, silicon, and organometallic compounds are used as initial products for plasma-chemical synthesis.

Figure 3 Scheme of the microwave installation for plasma-chemical synthesis:
I - power equipment (1 - microwave generator); II - main technological equipment (2 - plasma torch, 3 - reagent input device, 4 - reactor, 5 - heat exchanger, 6 - filter, 7 - powder collector, 8 - reagent dispenser, 9 - evaporator); III, IV - auxiliary technological equipment and control unit, respectively (10 - valves, 11 - rotameters, 12 - pressure gauges, 13 - gas purification system, 14 - scrubber, 15 - plasma gas input, 16 - carrier gas input, 17 - output gases)
Due to the peculiarities of plasma-chemical synthesis (non-isothermal process, the possibility of coagulation of particles, etc.), the size distribution of the obtained particles in most cases is quite wide.
Synthesis under ultrasonic treatment [ Applications of ultrasound to materials chemistry/ Suslick K.S., Price G.J. Annual Review Materials Science. - 1999. V.2., P. 295-326.]. This method is known as sonochemical synthesis, which is based on the cavitation effect of microscopic bubbles. During cavitation in a small volume, abnormally high pressure (up to 50 - 100 MN/m 2) and high temperature (up to 3000 K and above) develop, as well as huge heating and cooling rates (up to 10 10 K/s). Under conditions of cavitation, the bubble becomes, as it were, a nanoreactor. Using extreme conditions inside cavitation bubbles, many nanocrystalline (amorphous) metals, alloys, and refractory compounds (for example, Fe, Ni, and Co nanoparticles and their alloys from carbonyls, gold and copper colloids, Zr nanooxide, etc.) have been obtained.
Electrical explosion of wires [Nanopowders obtained using pulsed target heating methods/ Kotov Yu.A. promising materials. - 2003. No. 4., S. 79-81.]. It has long been noted that when current pulses with a density of 10 4 -10 6 A/mm 2 are passed through relatively thin wires, explosive evaporation of the metal occurs with condensation of its vapors in the form of particles of various dispersion. Depending on the environment, the formation of metal particles (inert media) or oxide (nitride) powders (oxidizing or nitrogen media) can occur. The required particle size and process productivity are controlled by the parameters of the discharge circuit and the diameter of the wire used. The shape of the nanoparticles is predominantly spherical; the size distribution of particles is normal-logarithmic, but rather wide. For nanoparticles with a size of 50-100 nm of metals such as Al, Cu, Fe and Ni, the plant productivity is 50-200 g/h with energy consumption up to 25-50 kWh/kg. Oxide nanopowders (Al 2 O 3 , TiO 2 , ZrO 2 , MgAl2O 4 , etc.) can also be produced, and after sedimentation treatment, the particle size can be very small (20-30 nm).
Some of the methods for obtaining nanopowders considered above in general form, of course, need to be detailed. The choice of the optimal method should be based on the requirements for the nanopowder and nanomaterial, taking into account economic and environmental considerations.
Consolidation methods. Almost all methods known in powder technology: pressing and sintering, various types of hot pressing, hot extrusion, etc. - applicable to ultrafine powders. In installations of the type shown in Figure 1, despite the use of fairly high pressing pressures (up to 2-5 GPa), even under vacuum conditions and with a small sample height (up to 1 mm), it is possible to obtain samples with a porosity of at least 10-15%. Ultrafine powders are characterized by low compressibility during pressing due to the significant influence of friction characteristics between particles. In the technology of pressing nanopowders at room temperatures, the use of ultrasonic vibrations is effective, which reduce the elastic aftereffect after the removal of the pressing load and somewhat increase the relative density of the pressed products, expanding the possibilities of their manufacture in the form of bushings and other shapes [Ultrasonic pressing of ultrafine ceramic powders / Khasanov O.L. . Izvestiya vuzov. Physics. - 2000. No. 5., S. 121-127.].
To eliminate residual porosity, heat treatment of pressed samples is necessary - sintering. However, as applied to the production of nanomaterials, the usual modes of sintering of powder objects do not allow preserving the original nanostructure. The processes of grain growth (recrystallization) and compaction during sintering (shrinkage), being diffusion-controlled, run in parallel, overlapping each other, and it is not easy to combine a high compaction rate with the prevention of recrystallization.
Thus, the use of high-energy consolidation methods, which involve the use of high static and dynamic pressures and moderate temperatures, makes it possible to delay grain growth to a certain extent.
Conventional modes of pressing and sintering ultrafine powders can be used to obtain nanostructured porous semi-finished products, which are then subjected to pressure treatment operations for complete consolidation. So, copper powders obtained by the condensation method, with a particle size of 35 nm with an oxide (Cu 2 O 3) film 3.5 nm thick after pressing at a pressure of 400 MPa and nonisothermal sintering in hydrogen up to 230 °C (heating rate 0.5 °C / min) acquired a relative density of 90% with a grain size of 50 nm [Fabrication of bulk nanostructured materials from metallic nanopowders: structure and mechanical behavior/ Champion Y., Guerin-Mailly S., Bonnentien J.-L. Script Materialia. - 2001. V.44. N8/9., P. 1609-1613.]. Subsequent hydrostatic extrusion led to the production of non-porous macrospecimens with high strength and plasticity (compressive yield strength 605 MPa, relative elongation 18%).
Grain growth during conventional sintering can be retarded using special non-isothermal heating modes. In this case, due to the competition between the mechanisms of shrinkage and grain growth, it is possible to optimize the compaction processes, eliminating to a large extent recrystallization phenomena - Kiev: Akademperodiika, 2001. - 180 p.]. Electrodischarge sintering, which is carried out by passing current through the sintered sample, and hot pressure treatment of powder objects (for example, forging or extrusion) can also contribute to the inhibition of recrystallization and can be used to obtain nanomaterials. Sintering ceramic nanomaterials under microwave heating, which leads to a uniform temperature distribution over the sample cross section, also contributes to the preservation of the nanostructure. However, the size of crystallites in the listed consolidation options is usually at the level of the upper limit of the grain size of the nanostructure, i.e. usually not lower than 50-100 nm.
Introduction
1 Emergence and development of nanotechnology
2 Fundamentals of nanomaterial technology
2.1 General characteristics
2.2 Consolidated materials technology
2.2.1 Powder technologies
2.2.2 Severe plastic deformation
2.2.3 Controlled crystallization from an amorphous state
2.2.4 Technology of films and coatings.
2.3 Technology of polymeric, porous, tubular and biological nanomaterials
2.3.1 Hybrid and supramolecular materials
2.3.2 Nanoporous materials (molecular sieves)
2.3.3 Tubular materials
2.3.4 Polymer materials
3 General characteristics of the application of nanomaterials
Conclusion
In the past few years, nanotechnology has come to be seen not only as one of the most promising branches of high technology, but also as a system-forming factor in the economy of the 21st century - an economy based on knowledge, and not on the use of natural resources or their processing. In addition to the fact that nanotechnology stimulates the development of a new paradigm of all production activities (“bottom-up” - from individual atoms - to the product, and not “top-down”, like traditional technologies, in which the product is obtained by cutting off excess material from a more massive workpiece) , it is itself a source of new approaches to improving the quality of life and solving many social problems in a post-industrial society. According to most experts in the field of science and technology policy and investment, the nanotechnology revolution that has begun will cover all vital areas of human activity (from space exploration to medicine, from national security to ecology and agriculture), and its consequences will be broader and deeper. than the computer revolution of the last third of the 20th century. All this sets tasks and questions not only in the scientific and technical sphere, but also before administrators at various levels, potential investors, the education sector, government bodies, etc.
Nanotechnology was formed on the basis of revolutionary changes in computer technology. Electronics as a holistic direction arose around 1900 and continued to develop rapidly throughout the past century. An exceptionally important event in its history was the invention of the transistor in 1947. After that, the heyday of semiconductor technology began, in which the size of the silicon devices being created was constantly decreasing. At the same time, the speed and volume of magnetic and optical storage devices continuously increased.
However, as the size of semiconductor devices approaches 1 micron, quantum mechanical properties of matter begin to appear in them, i.e. unusual physical phenomena (such as the tunnel effect). It can be confidently assumed that if the current pace of development of computer power is maintained, the entire semiconductor technology will face fundamental problems in about 5-10 years, since the speed and degree of integration in computers will reach some "fundamental" boundaries determined by the laws of physics known to us. Thus, the further progress of science and technology requires researchers to make a significant “breakthrough” to new operating principles and new technological methods.
Such a breakthrough can only be achieved through the use of nanotechnologies, which will make it possible to create a whole range of fundamentally new production processes, materials and devices, such as nanorobots.
Calculations show that the use of nanotechnologies can improve the basic characteristics of semiconductor computing and storage devices by three orders of magnitude, i.e. 1000 times.
However, nanotechnology should not be reduced only to a local revolutionary breakthrough in electronics and computer technology. A number of exceptionally important results have already been obtained, allowing us to hope for significant progress in the development of other areas of science and technology.
At many objects in physics, chemistry and biology, it has been shown that the transition to the nanolevel leads to the appearance of qualitative changes in the physicochemical properties of individual compounds and the systems obtained on their basis. We are talking about the coefficients of optical resistance, electrical conductivity, magnetic properties, strength, heat resistance. Moreover, according to observations, new materials obtained using nanotechnologies significantly outperform analogs of the micrometer scale in their physical, mechanical, thermal and optical properties.
Based on materials with new properties, new types of solar cells, energy converters, environmentally friendly products, and much more are already being created. Highly sensitive biological sensors (sensors) and other devices have already been created, which make it possible to talk about the emergence of a new science - nanobiotechnology and which have great prospects for practical application. Nanotechnology offers new opportunities for the micromachining of materials and the creation on this basis of new production processes and new products, which should have a revolutionary impact on the economic and social life of future generations.
2.1 General characteristics
The structure and, accordingly, the properties of nanomaterials are formed at the stage of their manufacture. The importance of technology as a basis for ensuring stable and optimal performance of nanomaterials is quite obvious; this is also important from the point of view of their economy.
The technology of nanomaterials, in accordance with the diversity of the latter, is characterized by a combination, on the one hand, of metallurgical, physical, chemical, and biological methods, and, on the other hand, of traditional and fundamentally new methods. So, if the vast majority of methods for obtaining consolidated nanomaterials are quite traditional, then such operations as manufacturing, for example, "quantum pens" using a scanning tunneling microscope, the formation of quantum dots by self-assembly of atoms, or the use of ion-track technology to create porous structures in polymer materials are based on fundamentally different technological methods.
The methods of molecular biotechnology are also very diverse. All this complicates the presentation of the fundamentals of nanomaterial technology, taking into account the fact that many technological details (“know-how”) are described by the authors only in general terms, and often the message is of an advertising nature. Further, only the main and most characteristic technological methods are analyzed.
2.2.1 Powder technologies
A powder is understood as a set of individual solid bodies (or their aggregates) in contact with small sizes - from a few nanometers to a thousand microns. With regard to the manufacture of nanomaterials, ultrafine powders are used as raw materials; particles with a size of not more than 100 nm, as well as larger powders obtained under conditions of intensive grinding and consisting of small crystallites with a size similar to those indicated above.
The subsequent operations of powder technology - pressing, sintering, hot pressing, etc. - are designed to provide a sample (product) of given shapes and sizes with the appropriate structure and properties. The totality of these operations is often called, at the suggestion of M.Yu. Balshina, consolidation. With regard to nanomaterials, consolidation should provide, on the one hand, almost complete compaction (i.e., the absence of macro- and micropores in the structure), and, on the other hand, preserve the nanostructure associated with the initial dimensions of the ultrafine powder (i.e., the grain size in sintered materials should be as small as possible and in any case less than 100 nm).
Methods for obtaining powders for the manufacture of nanomaterials are very diverse; they can be conditionally divided into chemical and physical, the main ones, of which, with an indication of the most characteristic ultrafine powders, are given in Table 1.
Table 1. The main methods for obtaining powders for the manufacture of nanomaterials
| Method | method variant | materials |
| Physical Methods | ||
| Evaporation and condensation | Vacuum or inert gas | Zn, Cu, Ni, Al, Be, Sn, Pb, Mg, Ag, Cr, MgO, Al 2 O 3 , Y 2 O 3 , ZrO 2 , SiC |
| in the reaction gas | TiN, AlN, ZrN, NbN, ZrO 3 , Al 2 O 3 , TiO 2 . | |
High Energy Destruction | Grinding | Fe-Cr, Be, Al 2 O 3 , TiC, Si 3 N 4 , NiAl, TiAl, AlN |
| Detonation treatment | BN, SiN, TiC, Fe, diamond | |
| electric explosion | Al, Cd, Al 2 O 3, TiO 2. | |
| Chemical Methods | ||
| Synthesis | Plasma chemical | TiC, TiN, Ti(C,N), VN, AlN, SiC, Si 3 N 4 , BN, W |
| laser | Si 3 N 4 , SiC, Si 3 N 4 -SiC | |
| Thermal | Fe, Cu, Ni, Mo, W, BN, TiC, WC-Co | |
| Self-propagating high temperature | SiC, MoSi2, Aln, TaC | |
| mechanochemical | TiC, TiN, NiAl, TiB 2 , Fe-Cu, W-Cu | |
| Electrochemical | WC, CeO 2 , ZrO 2 , WB 4 | |
| mortar | Mo 2 C, BN, TiB 2 , SiC | |
| cryochemical | Ag, Pb, Mg, Cd | |
| Thermal decomposition | Condensed Precursors | Fe, Ni, Co, SiC, Si 3 N 4 , BN, AlN, ZrO 2 , NbN |
| Gaseous precursors | ZrB 2 , TiB 2 , BN | |
Let us consider some of the methods for obtaining ultrafine powders.
Send your good work in the knowledge base is simple. Use the form below
Students, graduate students, young scientists who use the knowledge base in their studies and work will be very grateful to you.
Hosted at http://www.allbest.ru/
Nanotechnology is a field of fundamental and applied science and technology that deals with a combination of theoretical justification, practical methods of research, analysis and synthesis, as well as methods for the production and use of products with a given atomic structure by controlled manipulation of individual atoms and molecules.
The basis of all nanotechnologies is the ability of tetravalent elements (most often carbon) to form polyatomic and then multimolecular structures. Such structures most often have specific properties (depending on the composition, shape of the resulting molecule, and its other parameters) that are not inherent in any other known compounds, which makes them so interesting for science and opens up vast areas for the application of nanomolecules and nanotechnologies in general. nanotechnology technology material
For example, it turned out that nanoparticles of some materials have very good catalytic and adsorption properties. Other materials show amazing optical properties, such as ultra-thin films of organic materials used to make solar cells.
In turn, the ability of tetravalent elements, such as carbon, to form four bonds with other atoms is explained from the point of view of physics by the presence of four valence electrons in the external energy level.
Of course, it must be said that such an explanation does not quite solve the problem and is more chemical than physical. But if you drop further, you can see that everything is based on a physical phenomenon that explains the formation of bonds between atoms.
We also note that the modern description of the chemical bond is carried out on the basis of quantum mechanics, which is a branch of physics. A chemical bond is determined by the interaction between charged particles (nuclei and electrons). This interaction is called electromagnetic.
Methods for obtaining nanomaterials are divided into mechanical, physical, chemical and biological. Those. This classification is based on the nature of the process of nanomaterial synthesis. Mechanical production methods are based on the impact of large deforming loads: friction, pressure, pressing, vibration, cavitation processes, etc. Physical production methods are based on physical transformations: evaporation, condensation, sublimation, rapid cooling or heating, melt spraying, etc. (For completeness of classification and for reference) Chemical methods include methods, the main dispersing stage of which are: electrolysis, reduction, thermal decomposition. Biological methods of obtaining are based on the use of biochemical processes occurring in protein bodies.
Mechanical methods the emergence of a stress field and its subsequent relaxation do not occur during the entire time the particles are in the reactor, but only at the moment of particle collision and in a short time after it. The mechanical action is also local, since it does not occur in the entire mass of the solid, but where the stress field arises and then relaxes. Due to impulsivity and locality, large loads are concentrated in small areas of the material for a short time. This leads to the appearance of defects, stresses, shear bands, deformations, and cracks in the material. As a result, the substance is crushed, mass transfer and mixing of components are accelerated, and the chemical interaction of solid reagents is activated. As a result of mechanical abrasion and mechanical alloying, a higher mutual solubility of some elements in the solid state can be achieved than is possible under equilibrium conditions. Grinding is carried out in ball, planetary, vibration, vortex, gyroscopic, jet mills, attritors. Grinding in these devices occurs as a result of impacts and abrasion. A variation of the mechanical grinding method is the mechanochemical method. When a mixture of various components is finely ground, the interaction between them is accelerated. In addition, it is possible for chemical reactions to occur, which, if contact is not accompanied by grinding, do not occur at all at such temperatures. These reactions are called mechanochemical. In order to form a nanostructure in bulk materials, special mechanical deformation schemes are used, which make it possible to achieve large distortions in the structure of samples at relatively low temperatures. Accordingly, the following methods belong to severe plastic deformation:
High pressure torsion;
Equal-channel angular pressing (ECU-pressing);
All-round forging method;
Equal-channel angular hood (ECU-hood);
Hourglass method;
Sliding friction method.
At present, most of the results have been obtained by the first two methods. Recently, methods have been developed for obtaining nanomaterials using the mechanical action of various media. These methods include cavitation-hydrodynamic, vibration methods, shock wave method, ultrasonic grinding and detonation synthesis.
The cavitation-hydrodynamic method is used to obtain suspensions of nanopowders in various dispersion media. Cavitation - from lat. the words "emptiness" - the formation of cavities in a liquid (cavitational bubbles or caverns) filled with gas, steam or a mixture of them. During the process, cavitation effects caused by the formation and destruction of gas-vapor microbubbles in a liquid for 10-3 - 10-5 s at pressures of the order of 100 - 1000 MPa lead to the heating of not only liquids, but also solids. This action causes grinding of the solid particles.
Ultrasonic grinding is also based on the wedging effect of cavitation impacts. The vibrational method for producing nanomaterials is based on the resonant nature of effects and phenomena that provide minimal energy consumption during processes and a high degree of homogenization of multiphase media. The principle of operation is that any vessel is exposed to vibration with a certain frequency and amplitude.
Diamond nanoparticles can be obtained by detonation synthesis. The method uses the energy of an explosion, while reaching pressures of hundreds of thousands of atmospheres and temperatures of up to several thousand degrees. These conditions correspond to the region of thermodynamic stability of the diamond phase. The physical methods for obtaining UD materials include sputtering methods, evaporation-condensation processes, vacuum-sublimation technology, and solid-state transformation methods.
The method of atomizing a jet of melt with a liquid or gas is that a thin jet of liquid material is fed into a chamber, where it is broken into small drops by a stream of compressed inert gas or a jet of liquid. Argon or nitrogen are used as gases in this method; as liquids - water, alcohols, acetone, acetaldehyde. The formation of nanostructures is possible by quenching from a liquid state or by spinning. The method consists in obtaining thin strips by rapid (at least 106 K/s) cooling of the melt on the surface of a rotating disk or drum.
Physical methods. Evaporation-condensation methods are based on the production of powders as a result of a vapor-solid or vapor-liquid-solid phase transition in a gas volume or on a cooled surface.
The essence of the method lies in the fact that the initial substance evaporates by means of intense heating, and then cools rapidly. Heating of the evaporated material can be carried out in various ways: resistive, laser, plasma, electric arc, induction, ion. The evaporation-condensation process can be carried out in a vacuum or neutral gas environment. Electrical explosion of conductors is carried out in argon or helium at a pressure of 0.1 - 60 MPa. In this method, thin metal wires with a diameter of 0.1 - 1 mm are placed in a chamber and a high current is pulsed to them.
Pulse duration 10-5 - 10-7 s, current density 104 - 106 A/mm2. In this case, the wires instantly heat up and explode. The formation of particles occurs in free flight. Vacuum-sublimation technology for obtaining nanomaterials includes three main stages. At the first stage, the initial solution of the processed substance or several substances is prepared. The second stage - freezing the solution - aims to fix the uniform spatial distribution of the components inherent in the liquid in order to obtain the smallest possible size of crystallites in the solid phase. The third stage is the removal of solvent crystallites from the frozen solution by sublimation.
There are a number of methods for obtaining nanomaterials, in which dispersion is carried out in a solid substance without changing the state of aggregation. One of the ways to obtain massive nanomaterials is the method of controlled crystallization from an amorphous state. The method involves obtaining an amorphous material by quenching from a liquid state, and then crystallization of the substance is carried out under conditions of controlled heating. Currently, the most common method for obtaining carbon nanotubes is the method of thermal sputtering of graphite electrodes in arc discharge plasma.
The synthesis process is carried out in a chamber filled with helium under high pressure. During plasma combustion, intense thermal evaporation of the anode occurs, while a deposit is formed on the end surface of the cathode, in which carbon nanotubes are formed. The resulting numerous nanotubes have a length of about 40 μm. They grow on the cathode perpendicular to the flat surface of its end and are collected in cylindrical beams with a diameter of about 50 μm.
Nanotube bundles regularly cover the cathode surface, forming a honeycomb structure. It can be detected by examining the deposit on the cathode with the naked eye. The space between the nanotube bundles is filled with a mixture of disordered nanoparticles and single nanotubes. The content of nanotubes in the carbon precipitate (deposit) can approach 60%.
According to a small study I conducted on modern technologies that are being introduced into the production of clothing, I can say that some technologies are already actively used in the creation of materials for clothing and footwear, but as for bio- and nanotechnologies, so far information about such experiments, such as Olivia Ong , is very small and is quite rare on the web. I found about 10 examples mentioning the use of nanomaterials in making clothes.
…Unusual clothing designed by the Japanese research group Life BEANS…
…or German Evseevich Krichevsky, professor, doctor of technical sciences, honored worker of the Russian Federation, UNESCO expert, academician of RIA and MIA, laureate of the MSR State Prize, tells in an article for the nanonewsnet.ru website about his experience in implementing nanotechnologies in textile industries…
...Chinese scientists have created a nanofabric that cleans itself under the influence of solar radiation...
…Portugal is developing new materials and devices that are the latest innovation in the European research project DEPHOTEX…
And a few other mentions of other projects.
Unfortunately, despite some advances in the field of bio- and nanotechnology, and even specifically in the field of clothing, the resulting products remain prohibitively expensive for both the manufacturer and the buyer, so nanotech clothing is not yet ready to be produced in larger quantities. Today, this area is actively developing and remains a promising direction in the field of nanotechnology.
According to the forecasts of some scientists, the importance of the availability of high technologies in the future will be achieved through the search for rational methods and technologies for obtaining various nanomaterials and will ultimately lead to the widespread replacement of conventional materials with those obtained using high technologies.
The leader in the study of methods for obtaining nanomaterials is NSTU and TPU, in particular, the Department of Biotechnology on the basis of the Institute of Physics of High Technologies.
Hosted on Allbest.ru
...Similar Documents
General information about methods for obtaining nanoparticles. Basic processes of cryochemical nanotechnology. Preparation and dispersion of solutions. Biochemical methods for obtaining nanomaterials. Freezing liquid droplets. Supersonic outflow of gases from a nozzle.
term paper, added 11/21/2010
Study of the features of bulk nanostructured materials. History of development of nanotechnologies. Reasons for the widespread interest in nanotechnologies and nanomaterials. Methods for obtaining nanopowders. Plasma chemical and cryochemical synthesis. Cryotechnology products.
presentation, added 12/25/2015
Fullerite as a crystal of large carbon molecules Cn-fullerenes. Acquaintance with the main features of nanocrystalline materials, analysis of the advantages: high viscosity, increased wear resistance. Characterization of mechanical properties of nanomaterials.
abstract, added 05/20/2014
A group of methods for quantitative chemical analysis based on the use of electrolysis (electrochemical methods of analysis). Features of the electrogravimetric method, its essence and application. Basic equipment, internal electrolysis method.
abstract, added 11/15/2014
Nanocatalysis as a rapidly developing field of science that includes the use of nanomaterials as catalysts for various catalysis processes. Features of the production of nanoscale catalysts with 100% selectivity and high activity.
abstract, added 01/06/2014
Influence of mechanical activation on the geometrical parameters of dispersed materials. The main equipment used for sedimentation analysis of materials. Development of an installation for the study of materials, a feasibility study for this process.
thesis, added 04/16/2014
The concept and purpose of chemical methods for sample analysis, the procedure for their implementation and evaluation of effectiveness. Classification and varieties of these methods, types of chemical reactions carried out. Prediction and calculation of physical and chemical properties of different materials.
lecture, added 05/08/2010
Theoretical aspects of methods. The essence of testing materials for resistance to microscopic fungi and bacteria. Features of measurement of bioluminescence intensity and toxicity index. The main parameters for assessing the biostability of building materials.
abstract, added 01/13/2015
One of the most promising and promising directions in the development of modern science is nanotechnology. Research of nanocomposites from ceramics and polymers, nanocomposites containing metals or semiconductors. Possibilities of nanotechnologies.
abstract, added 01/26/2011
Study of chemical methods for obtaining powders: reduction of metal oxides and salts with solid or gaseous reducing agents, dissociation of carbonyls and unstable compounds, metallothermy. Extraction of iron from used car tires.
The course was developed by ANO "eNano" together with NUST "MISiS" and is aimed at students studying in the areas of study "Materials Science and Technology" and "Nanomaterials".
The eNano company is part of the RUSNANO group, developing courses and programs, as well as providing remote training for engineering and management personnel in the high-tech industry.
About the course
The course provides knowledge and practical skills in the field of physical and chemical foundations of the processes of obtaining nanoparticles and nanomaterials, helps to understand the relationship between the conditions for their formation and properties, introduces the basics of certification of nanoparticles and nanomaterials, problems and prospects for their practical application.
Based on knowledge about the phenomena occurring in homogeneous and heterogeneous systems with changes in temperature and pressure, as well as external mechanical influences, the student develops ideas about the physical and chemical foundations of the processes of obtaining nanoparticles and nanomaterials. The course tells about the "biographical" inheritance of properties by nanomaterials depending on the conditions for their production. As a result of mastering the course, the student will acquire the skills to perform calculations to determine the excess free energy of substances associated with an increase in their surface and defectiveness of the structure.
Format
The training takes place remotely. Weekly classes include:
watching thematic video lectures;
the study of illustrated textual materials, including 2-3 questions for self-examination for the assimilation of theoretical material;
performance of assessed test tasks after each section to control the assimilation of the material. Assignments count toward the certificate.
An important element of learning on the course is the completion of 2 individual tasks in the form of an essay for discussion on the course forum. It also provides final control testing for the entire content of the course.
Informational resources
Ryzhonkov D.I. etc. Nanomaterials. Tutorial. M.BINOM.Laboratory of knowledge. 2008, 280s. from ill.
Fahlman B. Chemistry of new materials and nanotechnology. Tutorial. M. ID Intellect. 2011, 317p. from ill.
Masuo Hosokawa, Kiyoshi Nogi, Makio Naito. Handbook of nanoparticle technology. M. Scientific world. 2013, 769s. from ill.
Requirements
To successfully master the course materials, students must first master:
"Chemistry",
"Phase equilibria and structure formation",
"Physical chemistry",
"Physical Properties of Solids",
"Processes for obtaining and processing materials",
"Diffusion and diffusion-controlled processes",
"Mechanical properties of materials",
"Theory of homogeneous and heterogeneous processes".
To take this course, students
Must know: fundamental sections of inorganic, organic and physical chemistry, their laws and methods, properties of chemical elements, compounds and materials based on them, patterns of structure formation and phase transformations, the influence of structural characteristics on the properties of materials, the main classes of modern materials.
Should be able to: carry out calculations of the main physico-chemical characteristics of reaction systems to determine the possibility and intensity of various transformations in them.
Must be proficient in: calculation of technological processes, use of methods of structural analysis and determination of the physical and physico-mechanical properties of materials, techniques for conducting experiments and their statistical processing.
Course program
Part 1. Classification of processes for obtaining nanoparticles. Physical and chemical bases of methods for obtaining nanosized powders (NP). NP certification.
- Gas-phase method for obtaining nanosized powders (NP). The main regularities of the formation of NPs by the method of evaporation and condensation.
- Condensation growth of nanoparticles (NPs). Coagulation and coalescence of NPs.
- Plasma recondensation method for obtaining nanoparticles.
- Plasma-chemical method for obtaining NP.
- Processes for obtaining nanoparticles (NPs) by precipitation of nanoparticles from solutions.
- Obtaining NP by thermal decomposition and reduction of metal-containing compounds.
- Mechanical method for obtaining NP. Mechanosynthesis.
- Electroexplosive method for obtaining NP. Comparative properties of NP obtained by different methods. Biographical inheritance of properties by them, depending on the method of obtaining.
- Certification of nanoparticles. Study of the composition, properties, dispersion.
Part 2. Fullerenes, carbon and non-carbon nanotubes.
- The history of the discovery of fullerenes. Fulleron structure formation mechanisms. Modified derivatives of fullerenes.
- Methods for producing carbon nanotubes (C-NT) (arc, laser-thermal, pyrolytic). Mechanisms of C-NT growth.
Part 3. Physical and chemical bases for obtaining bulk nanomaterials (NM).
- Classification of methods for obtaining bulk NM. Nanosized films and coatings deposited on a substrate. Chemical vapor deposition of nanostructured coatings (CVD).
- Physical deposition of nanostructured coatings from the vapor phase (PVD).
- Powder metallurgy of bulk NM. Molding NP.
- Sintering of nanopowders to obtain bulk nanomaterials.
- Severe plastic deformation as a way to obtain bulk NM. A method for obtaining bulk nanomaterials by controlled crystallization from an amorphous state.
Learning Outcomes
As a result of mastering the course "Processes for obtaining nanoparticles and nanomaterials" the student is able to:
use thermodynamic and kinetic analyzes of reaction systems to substantiate the most probable mechanism for the processes of obtaining nanoparticles and nanomaterials;
analyze the possibility of different methods of obtaining nanomaterials to form their desired properties and composition;
analyze the dispersity of nanomaterials obtained by various methods;
independently work with the literature to search for information about individual definitions, concepts and terms in the field of nanoparticles, including the processes for their production;
carry out calculations of the main indicators of the processes of obtaining nanoparticles and nanomaterials (equilibrium composition and yield of the target product);
prepare and carry out processes for obtaining nanoparticles and nanomaterials.
Formed competencies
(28.03.03 Nanomaterials PK3)
Ability to apply the main types of nanomaterials and nanosystems of inorganic and organic nature to solve production problems; have the skills to select these materials for given operating conditions;
(28.03.03 Nanomaterials PK2)
To be able to use in practice modern concepts of sciences about the properties of substances and materials during their transition to a nanoscale state (zero-, one-, two- and three-dimensional), about the influence of size on the properties of substances and materials, the interaction of nanomaterials and nanosystems with the environment;
(22.03.01 Materials science and technology of materials PC 1)
Ability to conduct supervised research and/or development work in the field of materials science and materials technology;
(22.03.01 Materials science and technology of materials PC 3)
Willingness to participate in the development of technological processes at the stage of development, introduction into production and testing of materials and products from them.
Limitations in the use of nanomaterials
It turned out that materials with nanosized grains are brittle. An important limitation for the use of nanostructured structural materials is their tendency to intergranular corrosion due to a very large volume fraction of grain boundaries. In this regard, they cannot be recommended for operation in conditions conducive to such corrosion. Another important limitation is the instability of the structure of nanomaterials, and, consequently, the instability of their physicochemical and physicomechanical properties. So with thermal, radiation, deformation, etc. influences, relaxation, segregation and homogenization processes are inevitable. When molding products from nanopowders, the problem of clumping (sticking together of nanoparticles) into agglomerates also arises quite acutely, which can complicate the production of materials with a given structure and distribution of components.
It should be noted that the commercial market is currently
the most widely represented nanomaterials are nanopowders
metals and alloys, nanopowders of oxides (silicon, iron, antimony, aluminum, titanium), nanopowders of a number of carbides, carbon nanofibers, fullerene materials.
Nanodispersed objects are obtained in the form of a sol, gel, concentrated dispersion or powder, thin film, nanoporous body. The range of methods for obtaining them is extremely wide. The existing methods for obtaining nanoobjects are classified according to the following criteria:
Synthesis strategy: obtaining can be based either on the dispersion process or on the condensation process - in foreign literature, these methods are divided into two groups: "top-down" - "top down", i.e. size reduction, grinding, and "bottom-up" - "bottom up", i.e. creation of nanostructures from smaller initial components, more precisely from atoms and molecules (both approaches are visually illustrated in Fig. 2.2);
The nature of the synthesis process (physical, chemical or biological);
Energy sources used in the synthesis process (laser, plasma, heating, freezing, mechanical, hydrothermal, combustion, etc.);
Medium in which nanoparticles or nanocrystals (NC) are formed (gas, liquid or solid).
The choice of a particular technology is determined by a number of factors, including the physical and chemical properties of the particles obtained, productivity, energy intensity of the process, environmental friendliness, etc.
The main methods for obtaining nanomaterials can be divided into a number of technological groups (Fig. 2.3): methods based on powder
metallurgy, methods based on the production of amorphous precursors, surface technologies (creation of coatings and modified layers with a nanostructure), methods based on the use of severe plastic deformation, and complex methods using several different technologies in series or in parallel.
 UCBrowser for PC is a worthy competitor for Google Chrome or not Uc browser fast mode
UCBrowser for PC is a worthy competitor for Google Chrome or not Uc browser fast mode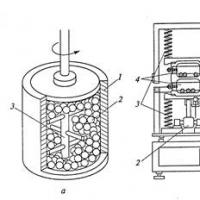 Basic technologies for obtaining nanomaterials
Basic technologies for obtaining nanomaterials How to tell the time in English?
How to tell the time in English? Presentation of the analytical report of the history teacher Participation in expert commissions
Presentation of the analytical report of the history teacher Participation in expert commissions Presentation of the analytical report of the history teacher
Presentation of the analytical report of the history teacher Presentation on the topic "atherosclerosis"
Presentation on the topic "atherosclerosis" History of number systems
History of number systems